New data from UNAIDS on the global HIV response reveals that during the last two years of COVID-19 and other global crises, progress against the HIV pandemic has faltered, resources have shrunk, and millions of lives are at risk as a result. The new report, In Danger, was launched ahead of the International AIDS Conference in Montreal, Canada.

Over the past two and a half years, the colliding AIDS and COVID-19 pandemics, along with economic and humanitarian crises, have placed the global HIV response under increasing threat. COVID-19 and other instabilities have disrupted health services in much of the world, and millions of students have been out of school, increasing their HIV vulnerability. Low- and middle-income countries have been challenged to respond as 60% of the world’s poorest countries are in debt distress or at high risk of it, and an estimated 75 to 95 million people have been pushed into poverty, an increase without precedent. As a result, the AIDS response has faced serious pressure while communities that were already at greater risk of HIV are now even more vulnerable.
In some parts of the world and for some communities, the response to the AIDS pandemic has shown remarkable resilience in adverse times, which has helped avoid the worst outcomes. However, global progress against HIV is slowing rather than accelerating: the latest data collected by UNAIDS show that while new HIV infections fell globally last year, the drop was only 3.6% compared to 2020—the smallest annual reduction since 2016. As a result, many regions, countries and communities are left to address rising HIV infections alongside other ongoing crises.
 To read the report, follow this link>>>.
To read the report, follow this link>>>.


 The United States Food and Drug Administration announced its first approval of a long-acting HIV prevention medication on 20 December 2021. The long-acting injectable cabotegravir (CAB – LA) is approved as a pre-exposure prophylaxis (PrEP) for adults and adolescents who are at risk of acquiring HIV sexually in the United States of America. Apretude is given first as two initiation injections administered one month apart, and then every two months thereafter. Patients can either start their treatment with Apretude or take oral cabotegravir (Vocabria) for four weeks to assess how well they tolerate the drug.
The United States Food and Drug Administration announced its first approval of a long-acting HIV prevention medication on 20 December 2021. The long-acting injectable cabotegravir (CAB – LA) is approved as a pre-exposure prophylaxis (PrEP) for adults and adolescents who are at risk of acquiring HIV sexually in the United States of America. Apretude is given first as two initiation injections administered one month apart, and then every two months thereafter. Patients can either start their treatment with Apretude or take oral cabotegravir (Vocabria) for four weeks to assess how well they tolerate the drug.

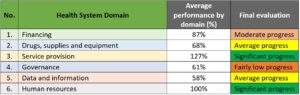

 The
The 
 The Global Fund to Fight AIDS, Tuberculosis and Malaria (the “
The Global Fund to Fight AIDS, Tuberculosis and Malaria (the “
 The next HepHIV conference will take place 5-7 May 2021 in a mixed face-to-face and virtual format involving participants from across community, public health and the health system.
The next HepHIV conference will take place 5-7 May 2021 in a mixed face-to-face and virtual format involving participants from across community, public health and the health system. The overall objective of
The overall objective of 
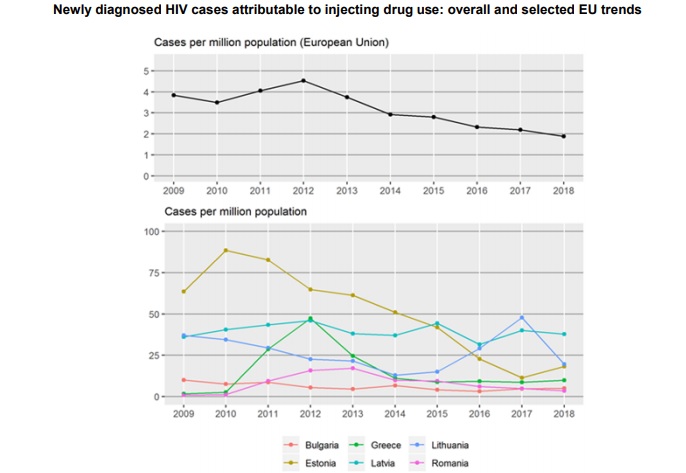
 Launched during European Testing Week (15–22 May), the report offers an overview of drug-related infectious diseases among PWID in Europe, including the prevalence and incidence of HIV and viral hepatitis. It also tracks progress on health targets and showcases successfully implemented evidence-based interventions. It underlines the need to ramp up prevention and testing and signals that European countries are lagging behind when it comes to treating hepatitis C virus (HCV) and HIV among PWID.
Launched during European Testing Week (15–22 May), the report offers an overview of drug-related infectious diseases among PWID in Europe, including the prevalence and incidence of HIV and viral hepatitis. It also tracks progress on health targets and showcases successfully implemented evidence-based interventions. It underlines the need to ramp up prevention and testing and signals that European countries are lagging behind when it comes to treating hepatitis C virus (HCV) and HIV among PWID. To read full report,
To read full report, 
 The rapid assessment is available
The rapid assessment is available 
 The Principal Recipient for Kosovo is the
The Principal Recipient for Kosovo is the