Excerpts from the EMCDDA press release
Testing for drug-related infectious diseases among people who inject drugs (PWID) is crucial if international health targets are to be met. This is among the conclusions of a new EMCDDA report Drug-related infectious diseases in Europe. The update, from the agency’s drug-related infectious disease network, stresses that early diagnosis through testing, and improving links to treatment and care, are crucial steps towards reaching global health goals.
 Launched during European Testing Week (15–22 May), the report offers an overview of drug-related infectious diseases among PWID in Europe, including the prevalence and incidence of HIV and viral hepatitis. It also tracks progress on health targets and showcases successfully implemented evidence-based interventions. It underlines the need to ramp up prevention and testing and signals that European countries are lagging behind when it comes to treating hepatitis C virus (HCV) and HIV among PWID.
Launched during European Testing Week (15–22 May), the report offers an overview of drug-related infectious diseases among PWID in Europe, including the prevalence and incidence of HIV and viral hepatitis. It also tracks progress on health targets and showcases successfully implemented evidence-based interventions. It underlines the need to ramp up prevention and testing and signals that European countries are lagging behind when it comes to treating hepatitis C virus (HCV) and HIV among PWID.
HIV and chronic viral hepatitis are highly prevalent among people who inject drugs, being transmitted through the sharing of injecting equipment, such as needles and syringes. Addressing the needs of this group is critical to achieve the UN Sustainable Development Goal of Good Health and Well-being (SDG 3), which calls for ending the AIDS epidemic and combatting viral hepatitis as a public health threat by 2030 (SDG 3.3).
Besides data which include SEE countries which are EU members, there is a small update from neighbouring countries within the Instrument for Pre-accession Assistance 7 and EU4Monitoring Drugs project:
The Instrument for Pre-accession Assistance (IPA) 7 technical cooperation project comprises six beneficiary countries: Albania, Bosnia and Herzegovina, Kosovo (1), Montenegro, North Macedonia and Serbia. Data on PWID and other key populations in the region are available from RDS seroprevalence studies: Albania, Kosovo and North Macedonia have conducted such surveys in the past 3 years; Bosnia and Herzegovina, Montenegro and Serbia are planning to collect data in 2020. There were no HIV-positive cases among PWID in recent surveys conducted in Kosovo or North Macedonia (Mikikj, 2017); older HIV prevalence estimates among PWID ranged between 0 % in Bosnia and Herzegovina in 2015 (Skocibusic et al., 2016) to 2 % in Serbia in 2013 (IPH Serbia, 2013). Most recent HCV infection prevalence estimates ranged from 23.8 % in Kosovo to 72 % in North Macedonia. All six beneficiaries are signatories of the Dublin Declaration.
 To read full report, follow this link>>>
To read full report, follow this link>>>




 The next HepHIV conference will take place 5-7 May 2021 in a mixed face-to-face and virtual format involving participants from across community, public health and the health system.
The next HepHIV conference will take place 5-7 May 2021 in a mixed face-to-face and virtual format involving participants from across community, public health and the health system. The overall objective of
The overall objective of 


 World Hepatitis Day (WHD) takes places every year on 28 July bringing the world together under a single theme to raise awareness of the global burden of viral hepatitis and to influence real change. In 2020 the theme is ‘Find the Missing Millions’. Have a look at the promotional video of the campaign in 2020
World Hepatitis Day (WHD) takes places every year on 28 July bringing the world together under a single theme to raise awareness of the global burden of viral hepatitis and to influence real change. In 2020 the theme is ‘Find the Missing Millions’. Have a look at the promotional video of the campaign in 2020 The
The  To inspire and challenge you with its essential stories, our colleagues from Correlation – Harm Reduction Network collected and published
To inspire and challenge you with its essential stories, our colleagues from Correlation – Harm Reduction Network collected and published 
 Launched during European Testing Week (15–22 May), the report offers an overview of drug-related infectious diseases among PWID in Europe, including the prevalence and incidence of HIV and viral hepatitis. It also tracks progress on health targets and showcases successfully implemented evidence-based interventions. It underlines the need to ramp up prevention and testing and signals that European countries are lagging behind when it comes to treating hepatitis C virus (HCV) and HIV among PWID.
Launched during European Testing Week (15–22 May), the report offers an overview of drug-related infectious diseases among PWID in Europe, including the prevalence and incidence of HIV and viral hepatitis. It also tracks progress on health targets and showcases successfully implemented evidence-based interventions. It underlines the need to ramp up prevention and testing and signals that European countries are lagging behind when it comes to treating hepatitis C virus (HCV) and HIV among PWID. To read full report,
To read full report, 
 European Testing Week offers partners across Europe the unique opportunity to unite to increase awareness of the benefits of early HIV and hepatitis testing among those who are at risk and promote increased access to testing. In 2019, more than 750 organisations from across 49 countries took part in ETW and thousands more people are now aware of their HIV and hepatitis status.
European Testing Week offers partners across Europe the unique opportunity to unite to increase awareness of the benefits of early HIV and hepatitis testing among those who are at risk and promote increased access to testing. In 2019, more than 750 organisations from across 49 countries took part in ETW and thousands more people are now aware of their HIV and hepatitis status.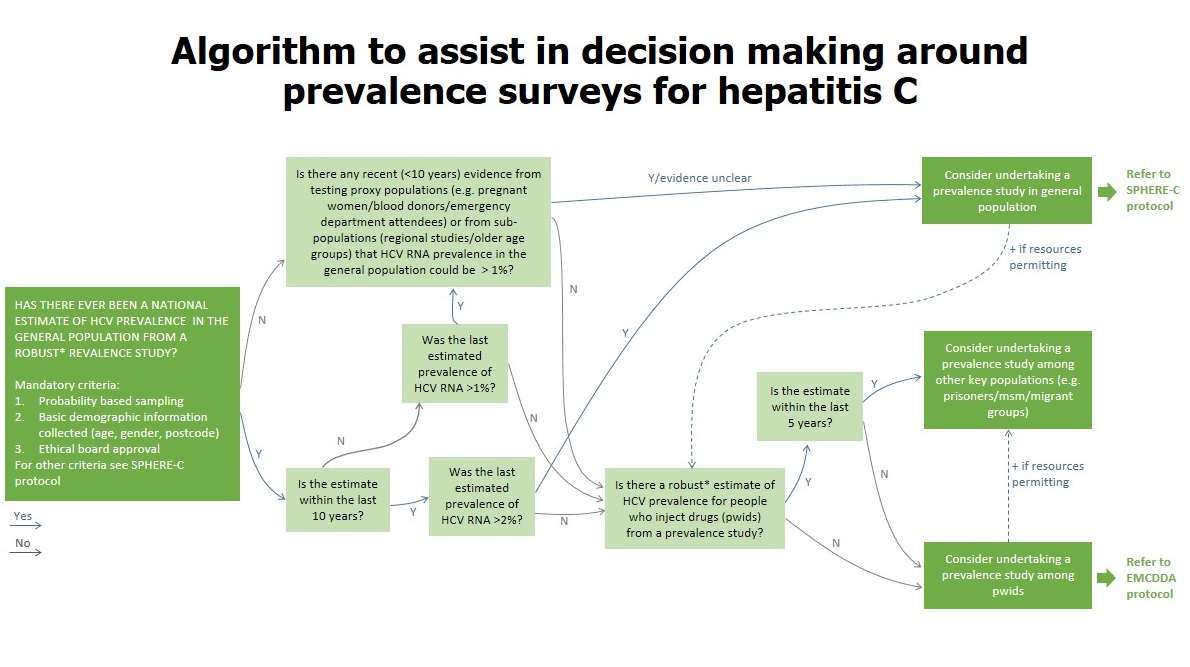
 To read and download the Protocol,
To read and download the Protocol,